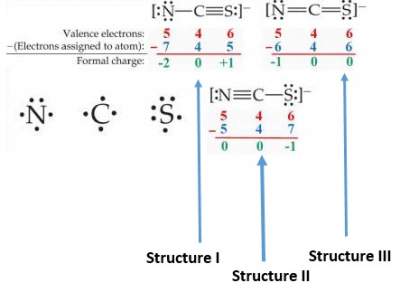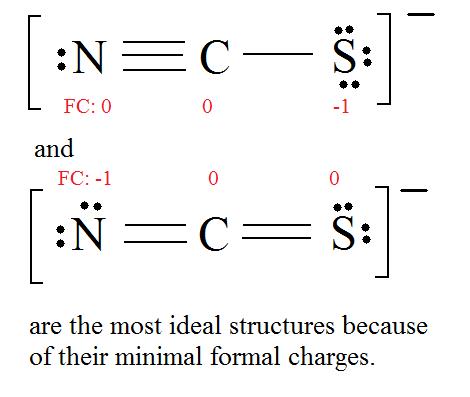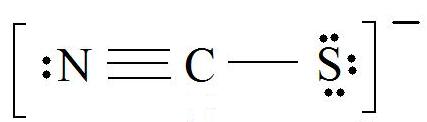
Catalyst 1. Draw the Lewis Structure for BCl3. 2. Draw the Lewis Structure for HCN. 3. Draw the Lewis Structure for PCl5. - ppt download

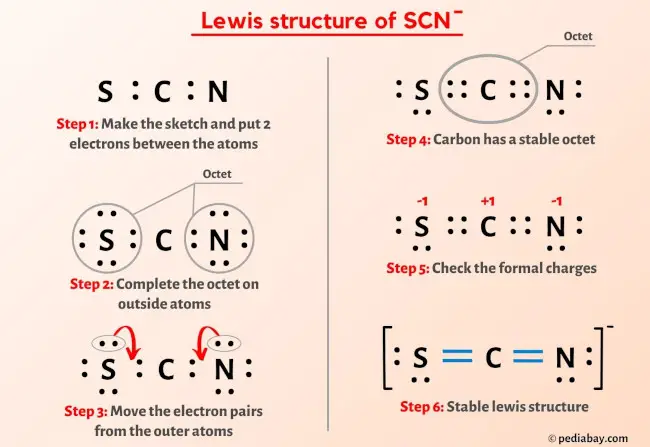
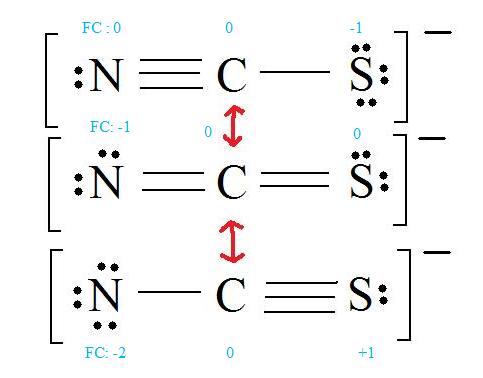
For thiocyanate ion SCN , three resonating structures are possible with the electron dot method as shown below:abCThe decreasing order of % contribution to the resonance hybrid is:A. Cannot be predicted B.
![SOLVED: Three possible structures that contribute to the resonance hybrid of the thiocyanate ion, [NCS]–, are shown below. Which resonance form will contribute the most to the resonance hybrid, and why? SOLVED: Three possible structures that contribute to the resonance hybrid of the thiocyanate ion, [NCS]–, are shown below. Which resonance form will contribute the most to the resonance hybrid, and why?](https://cdn.numerade.com/ask_previews/c2bdddd0-6e7c-480b-9952-d16f23d2c989_large.jpg)
SOLVED: Three possible structures that contribute to the resonance hybrid of the thiocyanate ion, [NCS]–, are shown below. Which resonance form will contribute the most to the resonance hybrid, and why?
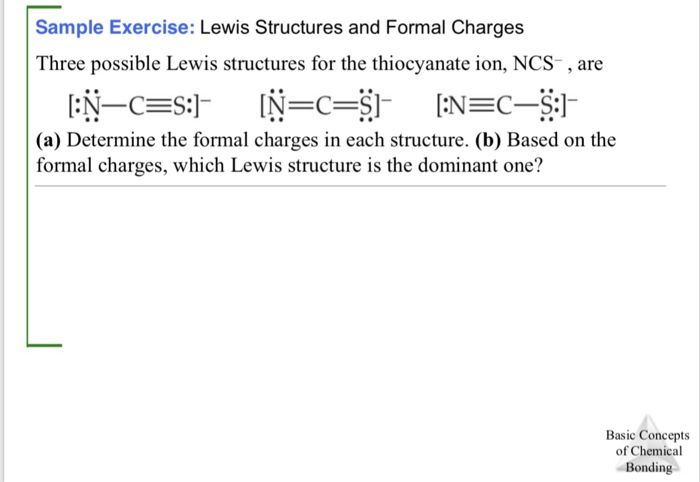
![SOLVED: Which of the following is the dominant Lewis structure for the CNS NCS ion? [N=C=S] None of the above [N=C=S:] [N=C- S:] SOLVED: Which of the following is the dominant Lewis structure for the CNS NCS ion? [N=C=S] None of the above [N=C=S:] [N=C- S:]](https://cdn.numerade.com/ask_images/6bbda53f956b4812a063fd1093699e59.jpg)
SOLVED: Which of the following is the dominant Lewis structure for the CNS NCS ion? [N=C=S] None of the above [N=C=S:] [N=C- S:]